Food and Drug Administration (FDA) |
Manufacturers and Importers may need to provide FDA information for food and drug related items before such items are imported and distributed in the United States. The FDA is responsible for ensuring that food sold in the United States is safe, wholesome, and properly labeled.
See Add/Modify PGA Information for directions on how to get to the FDA section.
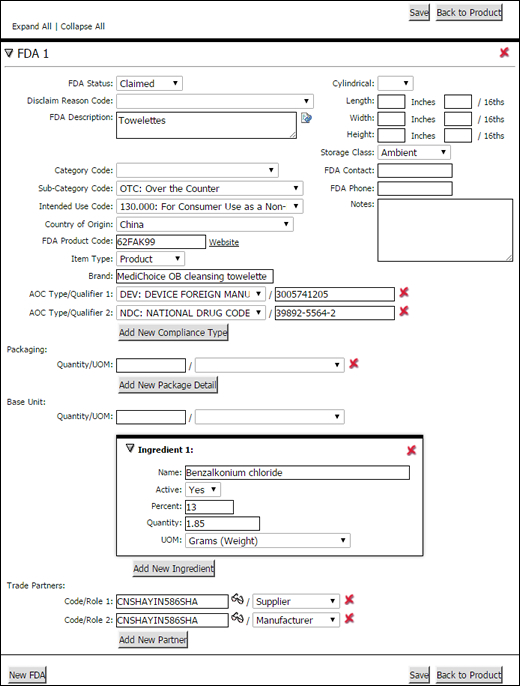
Figure 1: FDA Section
See the table below for field definitions in the FDA section. After you have finished filling out the required fields, click Save to save the record.
A list of the fields that appear in the FDA section:
Field |
Description |
|---|---|
Status |
If this PGA is non-applicable, select the status to Disclaimed, followed by a Disclaim Reason Code. |
Disclaim Reason Code |
If the PGA is non-applicable, select Disclaimed for the Status and then you must also select a disclaim reason code. This provides a clear and explicit flagging for these disclaim situations. |
Description |
The Description field has an icon with a blue arrow that you can click to populate the PGA Description field with whatever is listed in the Commercial Description in the main product screen. It is optional to use this feature, and a given PGA description does not necessarily need to match the main product Commercial Description. |
Category Code |
Select the category code from the drop-down list. This supports the U.S. Customs PGA requirements related to ACE. |
Sub-Category Code |
Select the sub-category code from the drop-down list. This supports the U.S. Customs PGA requirements related to ACE. |
Intended Use Code |
Select the intended use code from the drop-down list. This supports the U.S. Customs PGA requirements related to ACE. |
Country of Origin |
Select the origin country of the product from drop-down list. This is a required field. |
Product Code |
FDA
code for this product. Enter the code in the format "##XXX##".
Where # represents numbers and X represents letters. For
example, 13HAK37. |
Item Type |
Select the item type from the drop-down list. This supports the U.S. Customs PGA requirements related to ACE. |
Method Stored by |
Method used for storing the product. Select from the drop-down list with the following values:
|
Brand |
Brand name of the product. |
Affirmation of Compliance Codes (AOC) Type/Qualifiers |
Defines an FDA compliance code that applies to this product. Click Add New Compliance Type to add additional types. |
Compliance Code |
Specific commodity/condition code. Must be a valid FDA compliance. |
Compliance Code Qualifier |
If a compliance code is used, it must have a qualifier. |
Container DIMS Cylindrical, W/Dia, L, H |
Dimensions for an individual package for this product. The information is populated from Standard Package details if available. Includes three dimensions: width or diameter, height, and length. Unit of measure is always inches (IN). Select the Cylindrical check box if the package is cylindrical. |
Storage Class |
Select the storage class from the drop-down list. This supports the U.S. Customs PGA requirements related to ACE. |
Contact Name |
Contact name of the importer. |
Contact Number |
Contact phone number of the importer. |
Notes |
Space for notes. |
Packaging |
Describes packaging details of this product for the FDA. Click Add New Package Detail to add additional packaging information. |
Quantity |
Quantity for this package configuration. |
Unit of Measure |
FDA unit of measure for this packaging configuration. Must be a valid FDA unit of measure. |
Base Unit |
|
Quantity |
Base quantity. |
Unit of Measure |
Base unit of measurement. |
Ingredient |
Add ingredient information such as Name, Active, Percent, Quantity and UOM. Click Add New Ingredient to add additional ingredient information. |
Trade Partners |
Click Add New Partner to add additional trade partners. |
Code |
Enter
the supplier/vendor's Trade Partner ID by entering the
reference or clicking |
Role |
Select the role of the Trade Partner from the drop-down list. |
© 2021 Property of Expeditors International of Washington, Inc. and its subsidiaries.
Business Confidential and Proprietary. Reproduction by written authorization only.
- Created by: Expeditors Technical Communications Department -